By Feng Qirong
n 12 March, National Medical Products Administration published news that five new corona virus antigen self-test products were launched. Actually, in Nantong, there is a company which produces corona virus antigen quick test products, and its products were sold overseas as early as one and a half years ago. Up to now it has submitted application for registration to National Medical Products Administration, in the near future they will be put on domestic market officially.
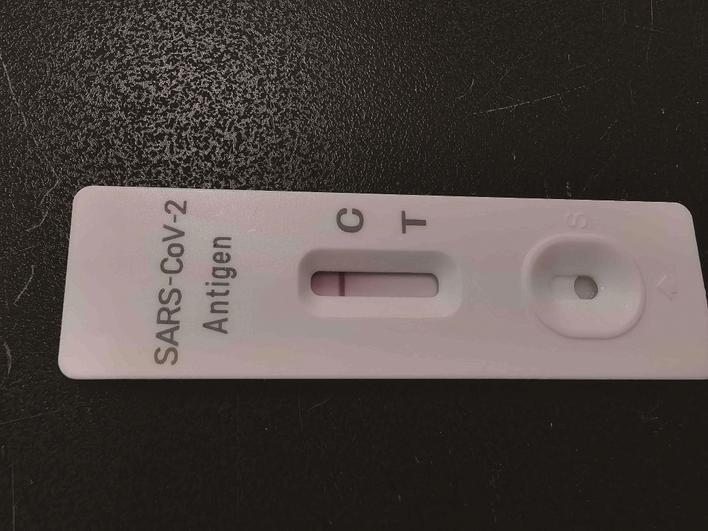
What do the antigen detection products look like? How to do a test? On 14 March afternoon, at Egens Biotechnology research institute of Nantong Egens Biotechnology Co., Ltd., one of the staff Chu Hui presented the approach and relevant dos and don’ts of the new corona virus antigen quick detection reagent kit’ producted by the company for the reporter on spot. ‘In the present, there are two new corona virus antigen quick detection kit products. The major difference is the difference in swab, one is nasal swab, the sample is taken from anterior nasal cavity; the other is nasopharyngeal swab, which is for nasal cavity sampling or oral sampling.’ Chu Hui said, ‘follow the steps in the manual, result can be obtained in 15 minutes.’
‘Quick detection reagent is fast and convenient. It can break restrictions on people and places. The test time is short. It is good for virus infection diagnosis and excluding suspicious cases.’ board chairman of Nantong Egens Biotechnology Co., Ltd. Ou Weijun introduced that as early as the second half of 2020, Egens Biotechnology quickly organized people, together with the experts of medical research institutions, researched and developed series of new corona virus (SARS-CoV-2) quick detection reagent kit successfully, including new corona virus antigen detection product, new corona virus antibody detection product, new corona virus combined antibody test product, and new corona virus nucleic acid detection product. In November 2020, the series products were launched successfully, sold to overseas markets in Germany, Brazil and Indonesia. The average annual sales reached 50 million.
In the present, the daily production capacity of Egens Biotechnology quick detection reagent kit is 3 million, the daily production capacity of new corona virus nucleic acid test kit is 500 thousand, the daily production capacity of nucleic acid amplification instrument is 400 sets, and more than 50 automatic reagent assembly lines were built. The company is in expanding stage now, it is expected to reach 10 million daily production capacity in two months.